Atomic structure teaching resources
Worksheets and lesson ideas to challenge students aged 11 to 16 to think hard about atomic structure and isotopes (GCSE and Key Stage 3)
Overview: never trust an atom, they make up everything! Atoms are the fundamental building blocks of matter and are built from protons, neutrons and electrons. A useful way to approach the teaching of atomic structure is to consider the changing models proposed by scientists throughout history. The image here could represent a progression in how students understand atomic theory, starting with a simple billiard ball model at Key Stage 2/3 and ending with the electron cloud model at A Level. As well as understanding that atoms are made from different subatomic particles, students also need to understand that these subatomic particles differ in their size, location and charge. It’s worth spending time explaining how atoms are held together by electrostatic forces of attraction between electrons and the nucleus (both directions) and by nuclear forces of attraction between nucleons.
Key concept: matter is made from discrete units called atoms. Atoms can differ from each other in the number of protons, neutrons and electrons they contain.
From big idea: all matter in the Universe is made from very small particles
Linked knowledge: periodic table, particles, bonding
Misconception [scientific idea]: the electrons in an atom orbit the nucleus like planets in our solar system orbit the sun [electron shells represent the specific amounts of energy electrons have, not where electrons are located]; the nucleus of an atom is equivalent to a nucleus in a cell [they are not analogous]. For a detailed explanation of alternative conceptions about atomic structure see this excellent publication from the RSC on chemical misconceptions.
Teaching resources
https://youtube.com/shorts/Q3DK1eeUGF0?feature=shared
Where to start?
Challenge students to tear a piece of paper into the smallest size possible. Then pose the question, ‘can this piece of paper be broken down further’? Explore ideas on the board and then introduce the idea of atoms, building on what students already know about particles. A fun thing to do is then ‘build an atom together’ using a range of artefacts to represent the different parts. Neutrons and protons should be represented by similar sized objects, with the same mass. Electrons can be represented by something with a tiny mass – such as a hair plucked form your head!
Particle theory (solids, liquids and gases) teaching resources
Resources to help students understand particles are found elsewhere
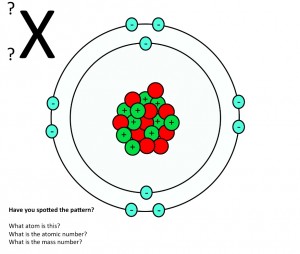
Mass number and atomic number – what do they mean?
GCSE activity on atomic number and mass number. Students work in pairs to deduce as many things as they can about atomic structure from diagrams showing three different atoms and one ion. The activity should lead to an understanding of what the atomic number and mass number mean. (PDF)
The language of atomic structure
GCSE worksheet on atomic structure key words. Key words used to describe atomic structure are written on the board. Students come up with a list of questions that their partner must answer by using the key words written on the slide. This activity promotes good discussion between students and supports meaning making of key terms. (PDF)
Electronic configuration of atoms
Whenever teaching atomic structure, it is vital students gain an understanding of scale and just how empty atoms are. This fantastic model helps students appreciate the scale of a hydrogen atom
GCSE worksheet on electronic configuration and the Periodic table. Students draw on the electronic configuration for the first 20 elements. They see for themselves how group number equals valence electron number. (PDF)
Follow this link for a GCSE activity where students have to decide if a model shows an atom or an ion.
Isotopes
Students need to understand and explain why isotopes have the same chemical properties but different physical properties. Heavy water can provide an excellent context to study isotopes. Ice cubes of heavy water sink in water. Perhaps get students to Predict, Observe and Explain what is happening in the video below from 6:14. Then move on to get students to calculate the relative atomic mass for hydrogen. Why is it not simply an average of the three naturally occurring isotopes 1H, 2H, and 3H i.e. 2 and not 1.00794?
The development of the model of the atom
GCSE worksheet on the development of the model of the atom. Students read about the development of the model of the atom throughout history and draw what they think the atom would have looked like at different points in time. Students then consider what was right and what was wrong about each model. The Phet animation of Rutherford’s scattering experiment will be useful to students. (PDF)
Thinking deeper
- If atoms are mostly empty space, why does it hurt when we bang into a wall?
- Why is it not possible to know precisely where an electron is?
- How is an atom similar and different to the solar system?