Osmosis teaching resources
Worksheets and lesson ideas to challenge students aged 11 to 16 to think hard about osmosis and osmoregulation (GCSE and Key Stage 3)
Overview: osmosis is the net (overall) movement of water molecules from an area of higher water concentration to an area of lower water concentration through a partially permeable membrane. Osmosis is really a special form of diffusion but with the added complexity that students need to to consider the role of the the solute and the membrane. Unlike simple diffusion, osmosis will not produce equal numbers of water particles on either side of the membrane. Instead, water will move to equalise the concentration of the solute on both sides of the membrane. The role of the membrane in stopping the solute particles from moving is therefore key. Spend time unpicking what partially permeable means – some simple analogy models can help here e.g. a sieve to show water particles passing through small gaps but not larger stones (the solute particles).
Key concept: water will move spontaneously through a partially permeable membrane into a region of higher solute concentration in order to equalise the solute concentrations on the two sides of the membrane. This movement of water into a cell exerts a force on the cell membrane which can be advantageous i.e. enable plants to stand upright or disadvantageous i.e. cause animal cells to burst.
From big idea: organisms are organised on a cellular basis and have a finite life span.
Linked knowledge: diffusion, concentration gradient, solute, solvent, solution, cell structure, particle model
Misconception [scientific idea]: water molecules move from A to B i.e. one direction only [water molecules will move in both directions, from A to B and from B to A but there will be a net movement of water molecules]; particles move from one place to another and stop [particle motion is random, independent and continuous]
Teaching resources
Where to start?
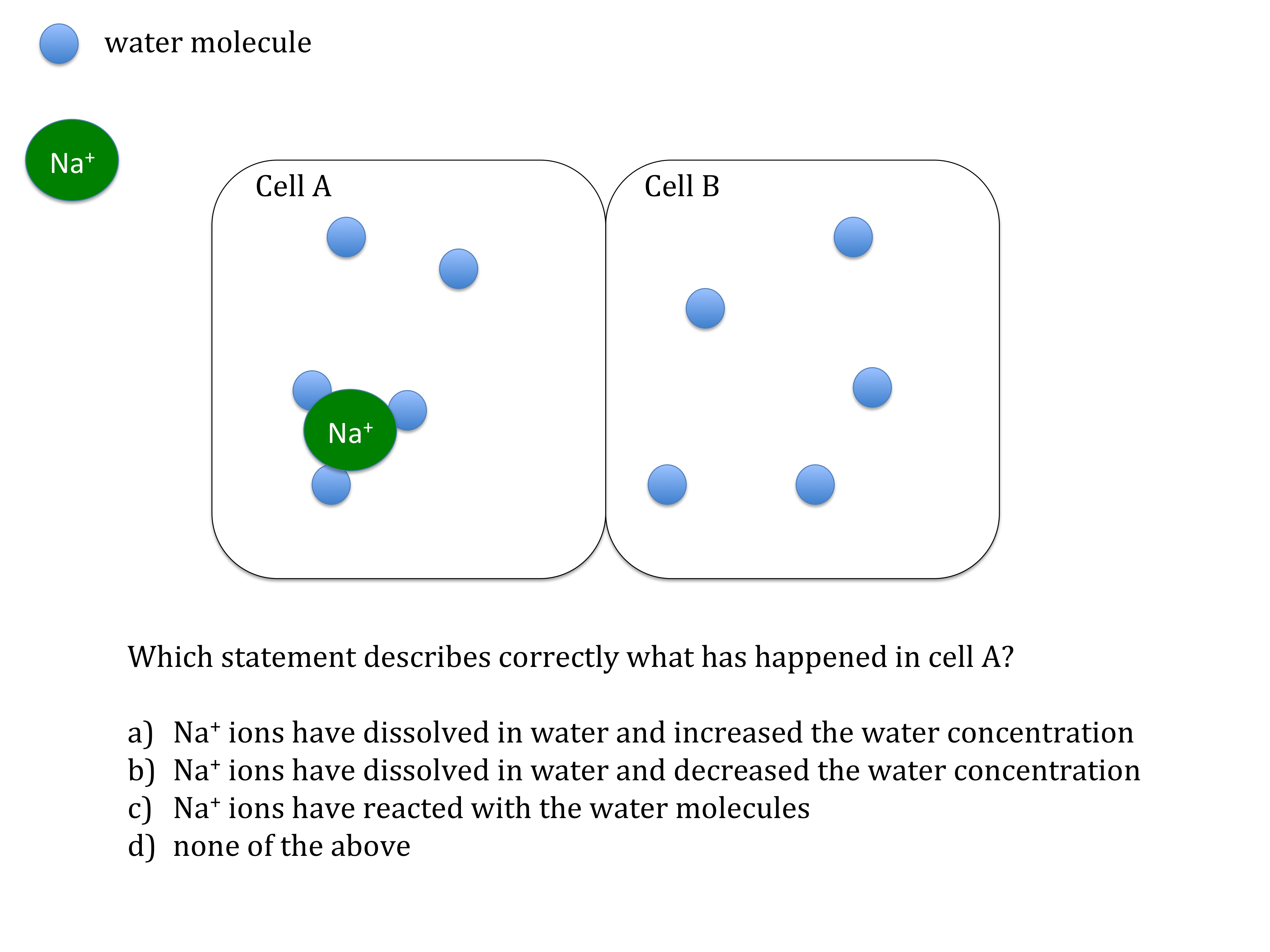
GCSE thought experiment about osmosis. Use this thinking task to get students to engage with the process of osmosis. This could be used at the start of the topic to challenge and motivate students to solve a problem. Whilst students may not arrive at the right answer, it will focus their thinking on the parts of the problem i.e. membrane, solute and water, making any explanations that follow more relevant and likely to stick. (PDF)
Observing osmosis
Two of my favourite ways to observe osmosis are the naked egg experiment and using gummy bears. For the naked egg experiment, place some uncooked eggs in vinegar overnight – this will remove the shell. Then place one egg in water and the other egg in a concentrated salt solution (8%) for 24 hours. Remove the eggs and observe what has happened. With the gummy bears (remember the theme tune!) just place them into different salt solutions and get students to observe what happens after 24hours. You can get them to take measurements before and after e.g. mass and length and calculate some percentage changes.
Investigating osmosis
Compare the isotonic point of sweet potato with white potato by placing potato disks in different concentrations of NaCl solution. Plot a graph of % change in mass versus concentration.
 Understanding osmosis – using MCQs
Understanding osmosis – using MCQs
GCSE diagnostic multiple choice questions on osmosis. Students work individually on each diagnostic multiple choice question and then discuss answers in pairs. Or, each question can be used within your existing lessons to check for understanding. Answers are in the notes section of the PPT file. It’s worthing checking out the further reading below on osmosis misconceptions – I think we teach most of them! (PDF)
Hypertonic, hypotonic and isotonic solutions and the effects of osmosis
GSCE worksheet on hypertonic, hypotonic and isotonic solutions. A simple context is set-up using a nurse who administers the wrong IV drip. This activity could be used to consolidate understanding around osmosis and the effects of hypotonic and hypertonic solutions on cells. (PDF)
Osmosis and osmoregulation in context
GCSE activity looking at how osmosis affects freshwater and marine organisms. Students work in small groups to apply their understanding of osmosis to explain different adaptations in marine and freshwater organisms. This activity requires students to have a good understanding of the principles of osmosis so they can apply their knowledge to new situations. (PDF)
Going deeper:
- Why does osmosis require a membrane?
- Suggest how the ‘antifreeze’ enables the wood frog to survive winter.
Further reading
Kosinski, R. J.; C. K. Morlok (2008). Challenging misconceptions about osmosis. Association for Biology Laboratory Education. 30: 63–87

Leave a Reply