Diffusion teaching resources
Worksheets and lesson ideas to challenge students aged 11 to 16 to think hard about diffusion (GCSE and Key Stage 3)
Overview: diffusion is the spreading out of of particles from an area of higher concentration to an area of lower concentration resulting in the net movement of a substance. Note that particles will diffuse in all directions and are constantly in motion. In biology, diffusion is responsible for the movement of oxygen particles from inside the alveolus into a red blood cell. Diffusion can only happen in solutions, liquids and gases where particles are free to move. When teaching diffusion for the first time it is worth considering what a rate is i.e. a change in the concentration of something/change in time.
Key concept: diffusion is a passive process (does not require additional energy) involving the net movement of a substance down its concentration gradient. The rate of diffusion can be increased by steepening the concentration gradient, increasing the temperature and increasing the surface area of the exchange surface.
From big idea: all matter in the Universe is made of very small particles
Prior knowledge: particle model (kinetic theory of matter), elements/mixtures/compounds
Misconception [scientific idea]: particles move in a given direction from A to B [particles move randomly in all directions from A to B and from B to A]; particles are only moving if the whole substance is moving [particles in a beaker or water are moving, even if the beaker of water isn’t – the particles are the substance]
Teaching resources
Where to start?
Start the lesson by frying some food at the front of the class. This should definitely stimulate some interest and get students thinking about particles mixing, and moving randomly from one place to another.
Observing diffusion
There are some great demonstrations to observe diffusion. Give students a stop clock and ask them to record when they first smell deodorant that has been sprayed in the middle of the class. If you also get students to raise their hands when they first smell the deodorant you will get a lovely ‘ mexican wave’ effect. I used to do this demo standing at the front of the class, but this introduces the misconception that particles move in one direction. Try adding lead nitrate and potassium iodide to a Petri dish of water, yellow lead iodide will form – this provides a great starting point for introducing the idea that particle size affects the rate of diffusion.
Measuring the rate of diffusion
GCSE practical to investigate how the rate of diffusion is affected by temperature. Students measure the rate of diffusion of food dye at different temperatures. Students also apply their understanding of diffusion and temperature to gas exchange in cold-water worms. (PDF)
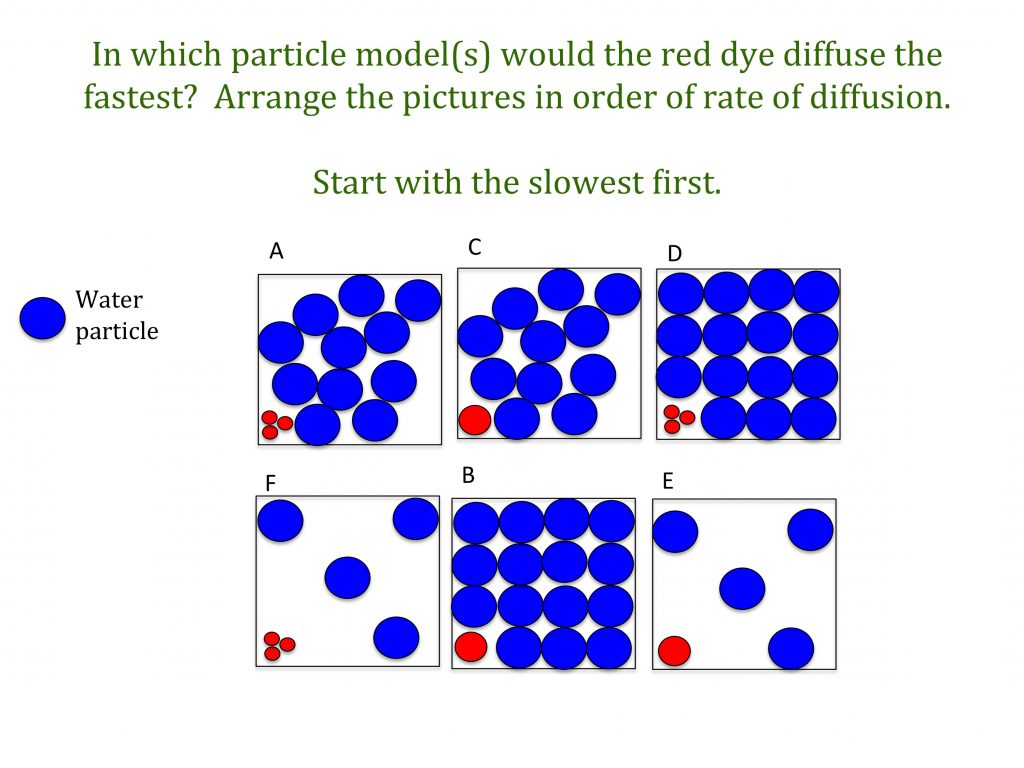
Factors affecting the rate of diffusion
Key Stage 3 worksheet on factors that effect the rate of diffusion. In this activity, students put a series of particle pictures in order of diffusion rate. The idea of this task is that it (hopefully!) will illicit the idea that the rate of diffusion depends on temperature and particle size. (PDF)
Thinking deeper
- Why don’t all particles at the same temperature diffuse at the same rate?
- Is diffusion the same as mixing? Explain your answer.
- How is the spread of fire through a forest similar and different to the diffusion of partciles?
Further reading