Electrolysis teaching resources
Worksheets and lesson ideas to challenge students aged 11 to 16 to think hard about electrolysis or molten compounds and electrolysis of aqueous compounds (GCSE and Key Stage 3)
Key concept: electrolysis is the splitting apart of an ionic compound into its elements using an electric current. Before we can start , the ions must be able to move – so melt or dissolve in water. The negative ions move to the anode where they lose their electrons to form the element. Positive ions move to the cathode, where they gain electrons to form the element. Electrons move though the wires. In this way, electrons are ripped from the negative ions (oxidation) and move into the circuit to replace the electrons given to the positive ions (reduction) – the power source is the electron pump.
Prior knowledge: ions, series circuits, elements, compounds, redox, solutions
Misconceptions [scientific idea]: NaCl(aq) exists as NaCl particles in solution [we get sodium and chloride ions, along with hydrogen and hydroxide ions]; electrons move in the electrolyte [ions move].
Teaching resources
Observing electrolysis
Students are amazed that by simply passing electricity through molten NaCl you can produce the metal sodium and the toxic gas chlorine – neither of which resemble the white solid you started with. Electrolysis of molten lead bromide works well in the classroom – use a fume cupboard.
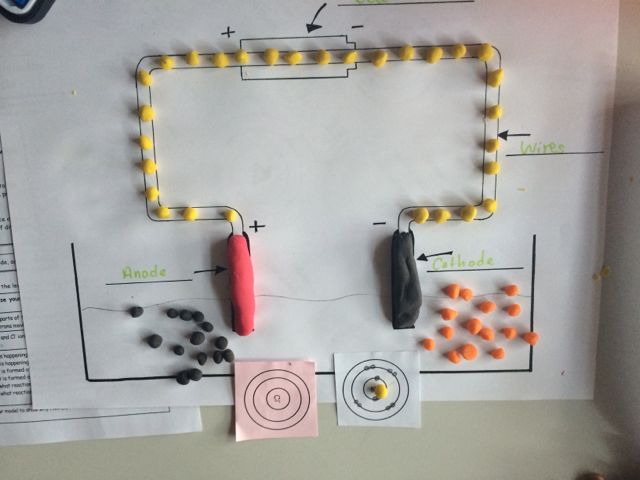
Creating a model of electrolysis to show electron movement
GCSE activity where students model electrolysis of molten sodium chloride. Students use success criteria on this worksheet and a blank template of an electrolysis circuit to model what is happening to the ions during electrolysis using Plasticine. (PDF)
Aluminium extraction
Click here to go to the page on the extraction of metals.
Electrolysis of aqueous solutions
GCSE activity on electrolysis of aqueous solutions. To understand electrolysis of aqueous solutions, students first need to understand exactly what ions are floating around in an aqueous solution. Then, it’s important they understand the less reactive element will get discharged – that’s because the more reactive element will be stable existing as an ion. Finally, they need to understand that the remaining solution contains a valuable product – sodium hydroxide. (PDF)
Further reading
An excellent summary of electrolysis of molten compounds by Jim Clark.
↵ Back to Chemistry teaching resources
