Equilibria teaching resources
Worksheets and lesson ideas to challenge students aged 11 to 16 to think hard about equilibrium, reversible reactions and the Haber process (GCSE and Key Stage 3)
Many students struggle with the concept of dynamic equilibrium, incorrectly assuming that equilibrium refers to a “balance” between reactants and products. The resources below strive to encourage students to understand equilibrium by starting with a simple physical change involving H2O(l) moving to H2O(g). It’s helpful to separate the concept of dynamic i.e. when rate of forward reaction equals rate of reverse reaction from equilibrium concentration i.e. when concentration of reactants and products remains constant. Students move on to apply their knowledge to chemical equilibria using Le Chatelier`s principle and finally think about compromise conditions in the context of the Haber process.
Where to start?
Heat a sample of hydrated copper sulphate in a test tube. Water vapour will be given off. Now add a few drops of water to form hydrated copper sulphate again. You could do this as a quick class practical. This can then lead on to a discussion about reversible and irreversible reactions.
Modelling a dynamic equilibrium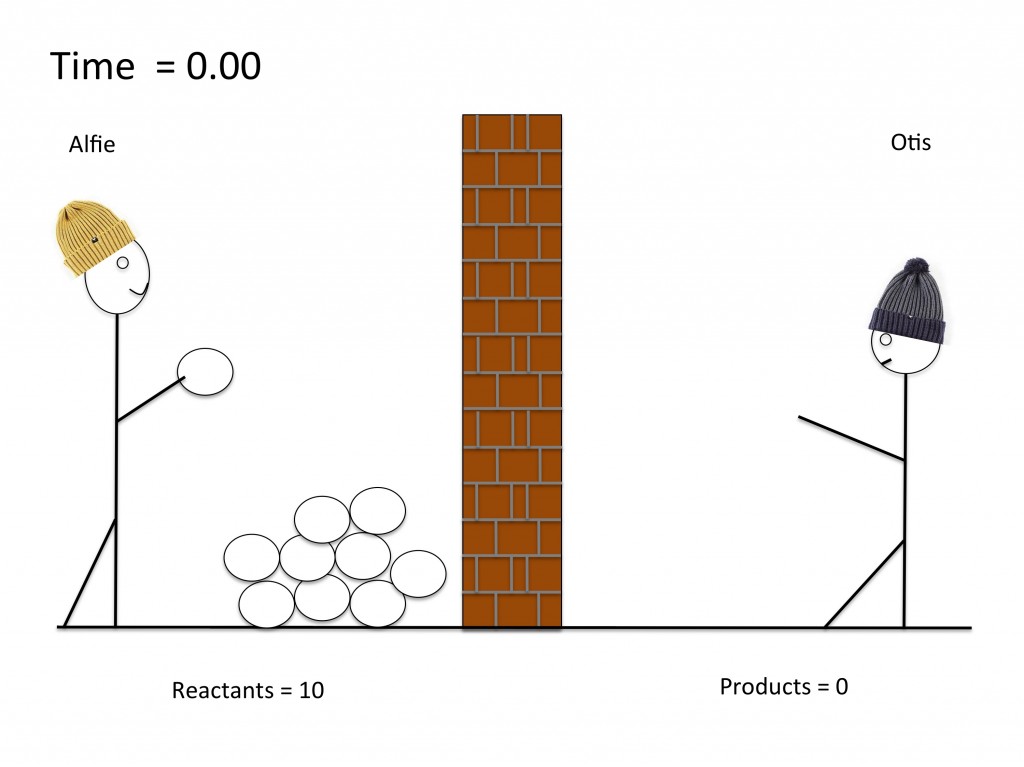
GCSE models to help understand equilibria. This simple model uses a snowball fight to show students how concentrations of reactants and products remain constant at equilibrium. This lesson can be further developed to help students understand the concept of position of equilibrium.
GCSE activity to understand dynamic equilibria. The worksheet describes a simple model of evaporation and condensation in a closed system. Students use the model to understand what happens at dynamic equilibrium to rates of forward and reverse reactions and to the concentration of reactants and products. (PDF)
Changing the position of equilibrium
GCSE activity on position of equilibrium and Le Chatelier’s principle. This is an excellent series of activities to get students thinking about Le Chatelier’s principle and provides a nice graphic to help explain what position of equilibrium actually means. (PDF)
GCSE worksheet on the chemistry of cola and Le Chatelier’s principle. Students consider how to make the perfect drink of cola. They use their knowledge of equilibria and how changes can affect the position of equilibrium according to Le Chatelier’s principle. (PDF)
Compromise conditions and the Haber Process
GCSE worksheet on the Haber process and compromise conditions. This activity gets students to think deeply about compromise conditions. They need to think about the balance between rate, yield and cost. (PDF)
Fritz Haber and fertiliser: the good, the bad and the ugly
The Haber process is perhaps the most important synthetic chemical reaction on Earth. The story of its founder, Fritz Haber, is an incredible one – a Jewish chemist who discovered the chemicals that were later used to kill members of his own family. A story that illustrates the good and evil science can do and is described brilliantly here by the BBC’s Radio Four in The Chemist of Life and Death. Get students to use this information to write an essay about Haber: the good, the bad, the ugly.
Thinking deeper
- Are all reactions reversible?
