Concentration and titrations teaching resources
Worksheets and lesson ideas to challenge students aged 11 to 16 to think hard about titration calculations, standard solutions and molarity (GCSE and Key Stage 3)
Show students what a mol dm-3 solution of NaCl actually is. Make it up in front of their eyes and all will become clear. Relate the quantities to the units: 1 mol dm-3 is simply means 1 mole in 1 litre. Now it makes sense! Never use triangles – these are hazards to learning and will only create problems in the future. If students can’t rearrange equations then teach them how to do it!
 Making a standard solution
Making a standard solution
Practical on how to make a standard solution. This step-by-step guide shows students how to make a standard solution of NaOH of know concentration. It encourages them to think about the importance of each practical step and not just blindly follow a method. There are many good reasons why you cannot produce an accurate primary standard solution of NaOH. Set your students the challenge of finding out why if they are finished. (PDF)
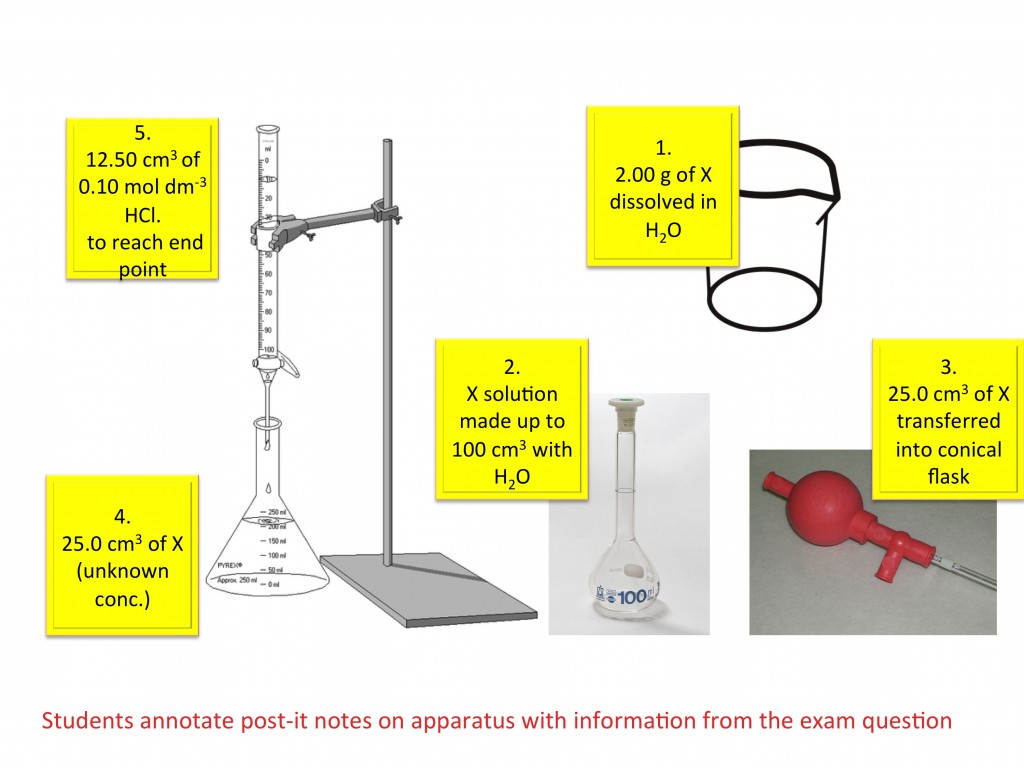
Titration calculations
This GCSE activity helps students understand titration calculations. Students are given a titration examination question and all the necessary apparatus. No chemicals are required. Students use Post-it Notes to label each item of apparatus with the reagent name and quantity it contains. They must also write a number on each Post-it Note to identify the order in which the equipment is used. Once students understand the method they can then begin the calculations. The use of Post-it Notes make it easy to assess student understanding. This activity was based on an idea by Dr Joao Metelo. (PDF)
