Acids, alkalis and salts teaching resources
Worksheets and lesson ideas to challenge students aged 11 to 16 to think hard about acids, alkalis and salts (GCSE and Key Stage 3)
For many students the acids and alkalis topic marks the beginning of their chemistry course. It is here that they find out that similar substances can be grouped together based on their chemical reactivity, and that chemicals can be detected using analytical techniques such as indicator paper. The topic also represents a great example of how scientific theories change and develop during history. I think it is worth using formulae early on so that students become familiar with symbols such as HCl, NaOH and H2SO4; familiarity breads confidence!
Where to start?
Have two or three beakers filled with colourless solutions – one water, one acid and one alkali. Ask students to think in their pairs how they could decide which of the three beakers contained an acid, an alkali and water. Hopefully this will then lead to a discussion about indicators. You can then add universal indicator at the end of the discussion to reveal the answer.
Making indicators
This is a great practical available from Learn Chemistry where students make their own indicator using cabbage.
The pH Scale
GCSE activity on the human pH scale. Students create a human pH scale by lining up in the classroom holding numbers -1 to 14. Other class members place different substances on the scale. This is a great exercise to assess prior learning – it could be used before any lesson on pH or acid/base chemistry. Slides showing pH of 0 and -1 are available if you feel brave enough to explain that pH is a measure of hydrogen ion concentration (pH=-log10[H+]) and can therefore be negative. (PDF)
Neutralisation reactions – the neutralisation challenge
GCSE activity on neutralisation reactions. This activity can be used with young (11-14) or older (14-18) students depending on whether you want students to think about dissociation and dissolving. Teaching neutralisation can be difficult as there are lots of simplifications that we make along the way e.g. H+ ions exist in solution (instead of H30+). It’s important that students understand that neutral solutions don’t always have a pH of 7. (PDF)
Reactions of acids
 GCSE and Key Stage 3 practical activity on reactions of acids. Students plan and carry out an investigation to identify a series of unknown substances. They must use their knowledge of reactions of acids. Students react unknown carbonates, metals, metal oxides and metal hydroxides with HCl. They test the products and use this information to identify the substances. It challenges students to problem-solve and think about reactions of acids rather than simply learning equations. (PDF)
GCSE and Key Stage 3 practical activity on reactions of acids. Students plan and carry out an investigation to identify a series of unknown substances. They must use their knowledge of reactions of acids. Students react unknown carbonates, metals, metal oxides and metal hydroxides with HCl. They test the products and use this information to identify the substances. It challenges students to problem-solve and think about reactions of acids rather than simply learning equations. (PDF)
Preparing a soluble salt
See our page on separating techniques for an activity to separate NaCl from a mixture.
Acids in context
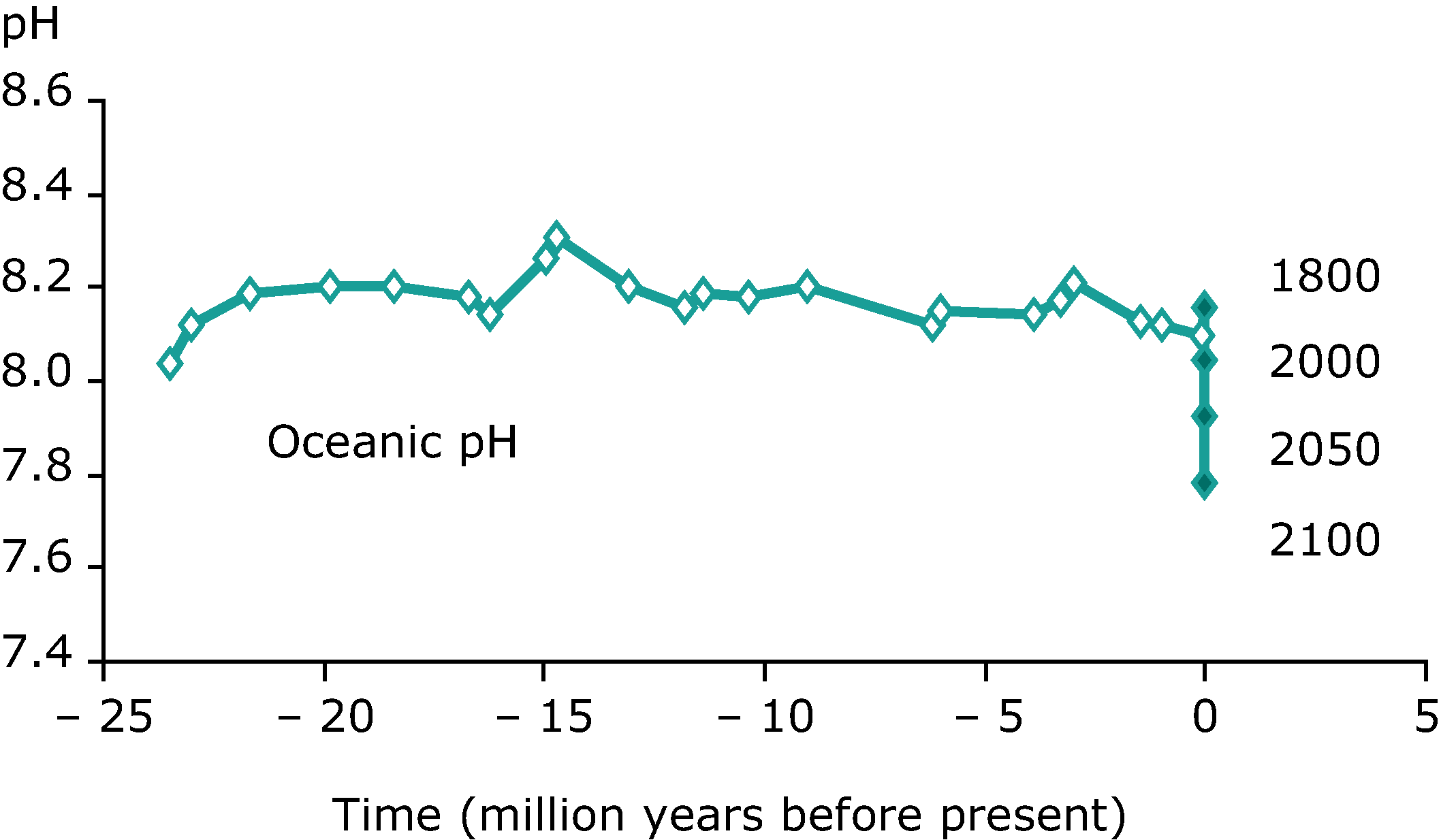
The acidification of oceans, caused by atmospheric carbon dioxide dissolving into the water, is one of the most significant challenges facing Earth. Even a small change in pH of the ocean can have devastating effects (importance of a log scale) on organisms. Humans, for example, can only tolerate small changes in blood pH of around 0.1. The RSC has an excellent article on ocean acidification.
